ATTACK ON TITAN Final Volume Vol.34 Comic Special Edition Manga inizio nuovo EUR 33,49 - PicClick IT
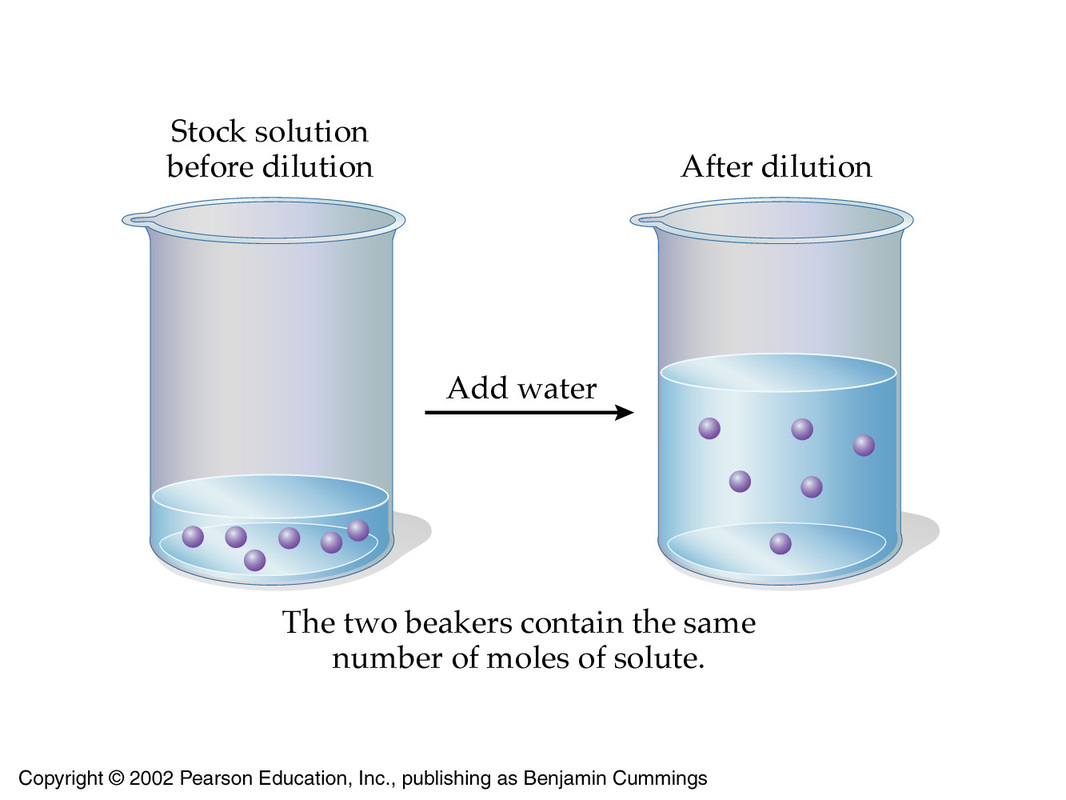
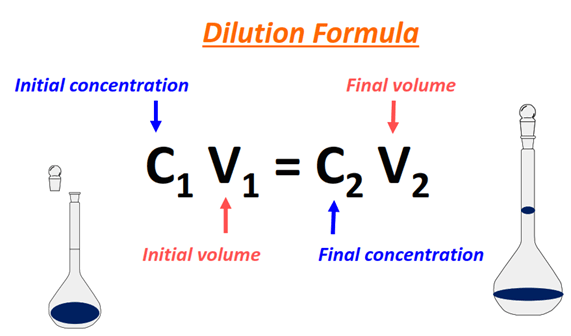
What is the final volume when 2.50 mL of a 11.0 M HCl solution is diluted to 0.100 M HCl solution? | Socratic

The final volume (in L) of one mole of an ideal gas initially {27}^{o}C and 8.21 atm pressure, it absorbs 420 cal of heat during a reversible isothermal expansion, is:
Calculate the final volume of reaction mixture when 10l CO and 10l O_2 are allowed to react to maximum possible extent. Pressure and temperature are same.

The final volume of a system is equal to the initial volume in a certain process. Is the work done - YouTube
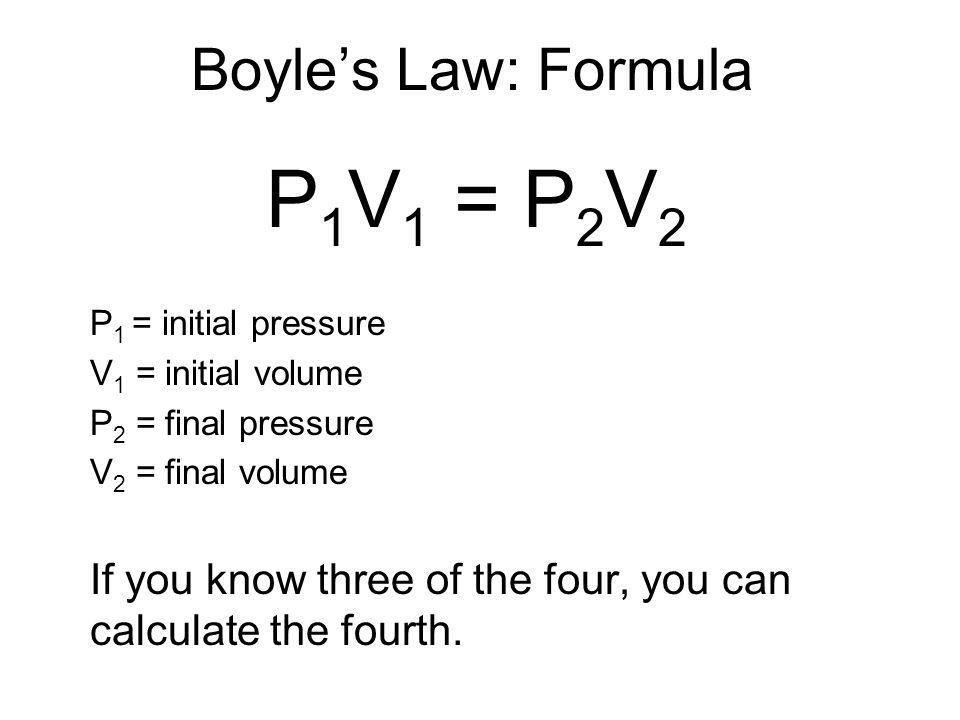
A "1.32 L" volume of gas has a pressure of "1.00 atm". What will the volume be if the pressure is increased to "30.0 atm"? | Socratic

SOLVED: If 4L of H2 gas at 1.43 atm is at standard temperature, and the pressure were to increase by a factor of 2/3, what is the final volume of the H2

Out of Oz: The Final Volume in the Wicked Years (Wicked Years, 4): Maguire, Gregory: 9780060859732: Amazon.com: Books
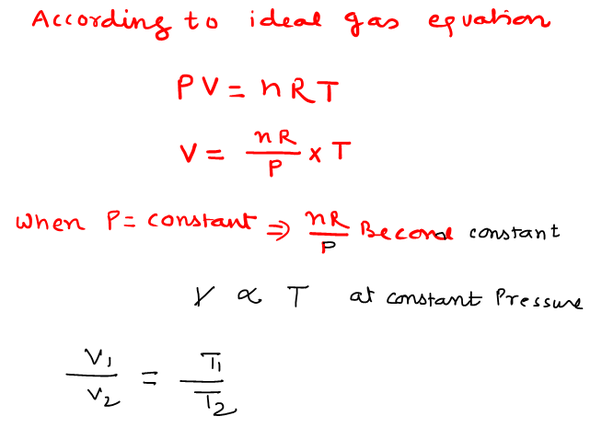
An ideal gas in a sealed container has an initial volume of 2.45 L. At a constant pressure, it is cooled to 19.00 °C where its volume is 1.75 L. What was